Toward a Hydrogen Society
Hydrogen is anticipated to be the next-generation energy source to replace fossil fuels. Various countries and regions, including Europe and Japan, have developed action plans aimed at creating a hydrogen society, and practical efforts are actively being undertaken. In some countries hydrogen businesses have even begun, signaling the start of serious commercial activity toward a hydrogen society.
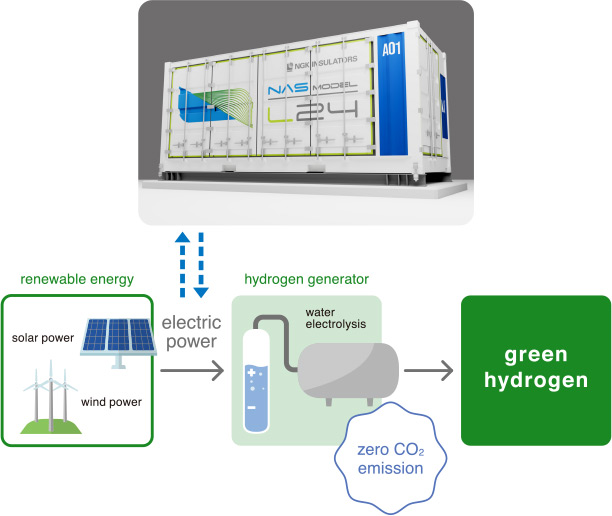
The main reasons hydrogen is gaining attention as a future energy source are its potential for significant CO2 reduction because it does not emit CO2 when combusted, and its ability to be produced from a wide range of materials. Hydrogen can be manufactured through water electrolysis, as well as from natural gas, coal, oil, alcohol, and even biomass and waste plastics. Additionally, hydrogen's versatility for applications in power generation, mobility and manufacturing, along with its ease of storage and transportation, are also considered advantages.
The Key to Decarbonization
The most effective way to reduce CO2 is to utilize hydrogen produced by water electrolysis using electricity from renewable energy sources such as solar and wind power. Hydrogen produced with renewable energy is called green hydrogen. Green hydrogen promises to be carbon-free from production through to utilization, making it a crucial component in achieving the goal of net-zero greenhouse-gas emissions by 2050.
To commercialize green hydrogen, it is essential to electrolyze water with as high an efficiency as possible and to do so stably. One technology that supports this goal is the large-capacity storage battery known as the NAS (sodium-sulfur) battery. NAS batteries, which allow for long-duration continuous discharge, can store renewable energy for use when needed. This capability enables stable hydrogen production through continuous electrolysis at a constant output.
NAS Batteries support stable green hydrogen production
Renewable energy generation is often affected by weather conditions, so a mechanism to store electricity generated during favorable weather conditions and supply it as needed is essential for stable power supply. A reliable large-capacity energy storage system is indispensable for balancing supply and demand of renewable energy.
Large-capacity storage batteries for stabilizing power supply
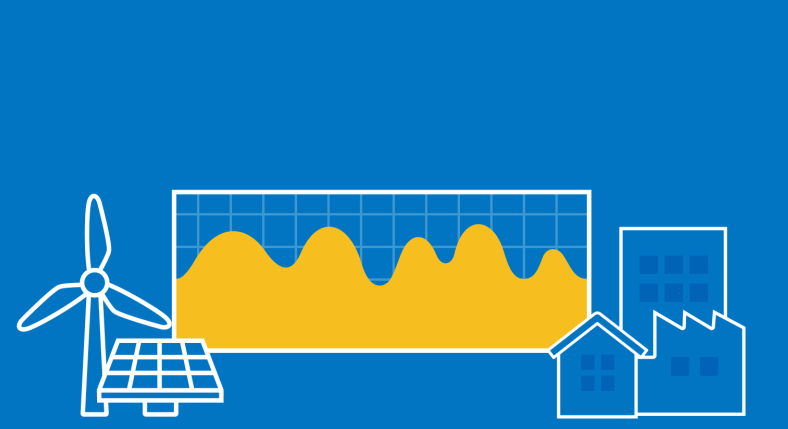
Renewable energy involves
large output fluctuations

NAS battery charging/discharging to
stabilize power supply
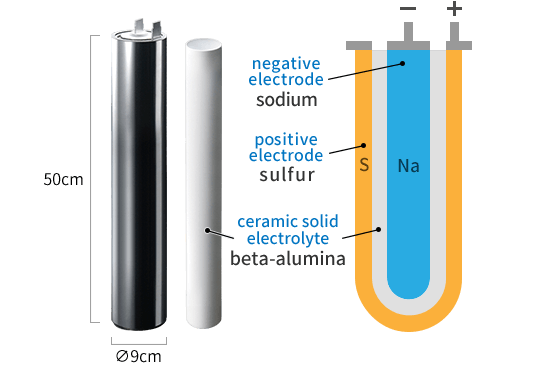
The NAS battery, the world’s first megawatt-class large-capacity storage battery, uses sodium (Na) for the negative electrode and sulfur (S) for the positive electrode, with β-alumina ceramic as the fixed electrolyte for charging and discharging cycles. With its long lifespan and high energy density, when paired with large-scale solar power installations, it can provide stable power supply that covers the entire lifespan of power generation equipment.
NAS Battery Cell
NAS Batteries: Optimal for Large-Scale Electrolysis
There are several methods for water electrolysis, including alkaline and PEM (Proton Exchange Membrane) types. Alkaline electrolysis is well-suited for stable, large-scale hydrogen production, while PEM electrolysis offers compact equipment and excellent responsiveness to fluctuations in power input. NAS batteries have a high affinity with alkaline electrolysis systems, becoming an ideal infrastructure for large-scale hydrogen production.
The NAS batteries by NGK Insulators are well-suited for long-term power storage with high output and a lifespan of 20years, enabling stabilization of renewable energy power and efficient hydrogen production at a lower cost. For this reason, they are expected to be utilized in green hydrogen production. NAS batteries will play a significant role in the realization of a hydrogen-based society and will push to make green hydrogen production a reality worldwide.
*NAS and the NAS logo are trademarks of NGK INSULATORS, LTD., registered in the U.S. and other countries.
NAS battery

Writer
Furukori Etsuko
Science and Technology Journalist
Furukori Etsuko graduated from the University of Tokyo with a bachelor's degree in chemistry and liberal arts. While working for a pharmaceutical company, she began writing articles for science magazines and discovered the joy of interviewing researchers and engineers. Since then, she has been writing, researching, planning and editing articles on science, technology and related topics. She is an MIT Science Journalism Fellow and a founding member of Sci-Tech Communications.


